Atropine and pralidoxime are the antidotes for cholinergic toxicity, reversing symptoms by blocking receptors and reactivating acetylcholinesterase.
Cholinergic toxicity is also known as cholinergic toxidrome. Cholinergic toxicity causes include excessive stimulation of your parasympathetic nervous system due to an overabundance of acetylcholine in your synapses. cholinergic toxicity is commonly caused by exposure to organophosphate and carbamate insecticides, certain medications, nerve agents, and sometimes poisonous mushrooms.
It leads to cholinergic toxicity symptoms:
- Excessive salivation
- Lacrimation (tearing)
- Bradycardia (slow heart rate)
- Bronchospasm
- Muscle weakness
- Confusion
- Paralysis
The management of cholinergic toxicity hinges on the rapid administration of specific antidotes aimed at reversing the toxic effects. The two main antidotes used to treat cholinergic toxicity are atropine and pralidoxime (2-PAM). Understanding how these antidotes work and when to use them is crucial for your effective treatment.
Cholinergic Toxicity Mnemonic
A commonly used mnemonic to remember the muscarinic symptoms of cholinergic toxicity is SLUDGE, which stands for Salivation, Lacrimation, Urination, Diaphoresis/Diarrhea, Gastrointestinal cramps, and Emesis. Another helpful mnemonic is DUMBBELS, representing Diarrhea, Urination, Miosis, Bradycardia, Bronchospasm, Emesis, Lacrimation, and Salivation. These mnemonics encapsulate the key features of cholinergic toxidrome, aiding in quick clinical recognition and recall of the excessive parasympathetic effects caused by acetylcholine accumulation
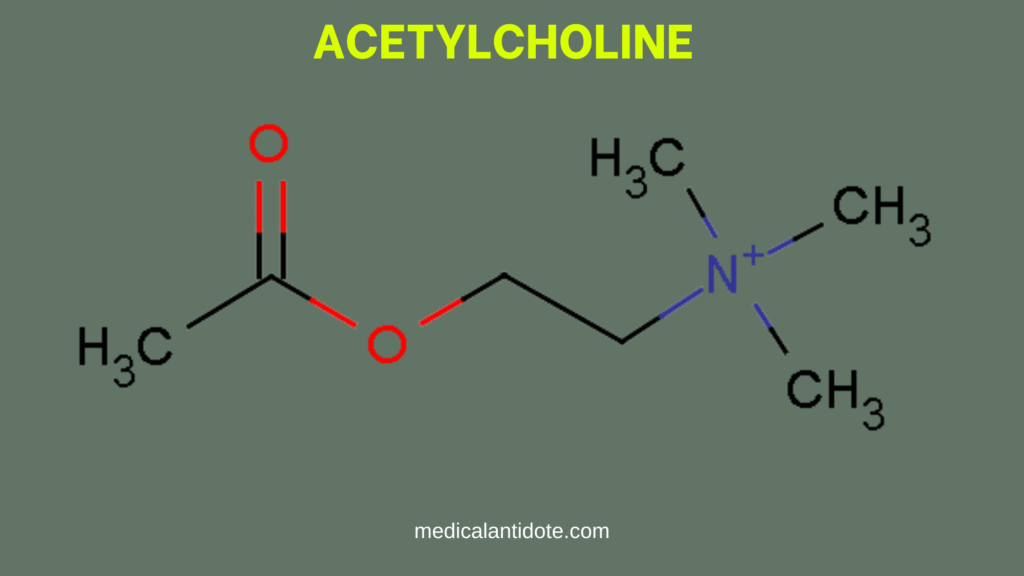
Excess Acetylcholine= Cholinergic Toxicity
Acetylcholine is a vital neurotransmitter in the parasympathetic nervous system, responsible for muscle contractions and glandular secretions. In cholinergic toxicity, excessive acetylcholine overwhelms the system, binding to both muscarinic and nicotinic receptors, resulting in an overstimulation of these receptors.
Key Cholinergic Toxicity Symptoms Include:
- Increased salivation and sweating
- Miosis (small pupils)
- Bronchorrhea and bronchospasm (excessive lung secretions and airway constriction)
- Bradycardia and hypotension
- Muscle twitching, weakness, and paralysis
- Gastrointestinal symptoms like vomiting and diarrhea
- Confusion and seizures in severe cases
These characteristic symptoms are well documented in clinical toxicology sources on cholinergic toxicity and organophosphate poisoning.
Cholinergic Toxicity Treatment
Cholinergic toxicity is treated using Atropine and Pralidoxime (2-PAM)
Atropine: The Physiologic Cholinergic Toxicity Antidote
Atropine is the cornerstone of treatment for cholinergic toxicity. It works as a competitive antagonist at muscarinic acetylcholine receptors. By occupying these receptors, atropine blocks the effects of excess acetylcholine, thereby reducing the muscarinic symptoms such as bronchorrhea, bronchospasm, bradycardia, and excessive glandular secretions.
How Atropine Is Used:
- Administered intravenously
- Initial adult dose ranges from 2 to 5 mg, repeated every 3 to 5 minutes as needed
- The dose may be doubled repeatedly until signs of atropinization appear (dry skin and mucosa, increased heart rate, reduced secretions, no bronchospasm, and dilated pupils)
- High doses may be required in severe poisonings, sometimes several hundred milligrams administered over days until the patient stabilizes
Atropine does not affect nicotinic receptors at the neuromuscular junction, so muscle weakness and paralysis caused by nicotinic receptor overstimulation are not relieved by atropine alone. This mechanism and clinical protocol for atropine administration are outlined in detail in clinical treatment guides.
Pralidoxime (2-PAM): The Biochemical Cholinergic Toxicity Antidote
Pralidoxime complements atropine therapy by targeting the underlying cause of toxicity—the inhibition of acetylcholinesterase, the enzyme responsible for breaking down acetylcholine.
Mechanism of Action:
- Reactivates acetylcholinesterase by removing the organophosphate compound bound to it (Discover Organophosphorous Poisoning Antidote)
- Provides endogenous anticholinergic effects
- Detoxifies unbound organophosphates
- Particularly effective against nicotinic symptoms such as muscle weakness, including respiratory muscle weakness
Pralidoxime is typically given intravenously, with an FDA-approved dose of 1 to 2 grams infused over 30 minutes. The dose may be repeated after one hour if muscle weakness persists, and sometimes a continuous infusion is warranted in severe cases.
However, pralidoxime’s effectiveness diminishes after a process called “aging,” where the acetylcholinesterase-organophosphate bond undergoes a conformational change, rendering the enzyme irreversibly inhibited and resistant to reactivation by pralidoxime. Therefore, early administration is critical for maximum efficacy. Due to variability in responses, pralidoxime is generally administered in all but mild cases of organophosphate poisoning. This treatment approach is supported by extensive organophosphate toxicity literature and clinical case studies.
Supportive Therapy and Additional Measures
Besides atropine and pralidoxime, supportive care is a vital component of treatment for cholinergic toxicity.
Oxygen and Respiratory Support:
- Since bronchorrhea and bronchospasm can impair breathing, oxygen therapy is essential.
- Mechanical ventilation may be necessary if respiratory muscles are paralyzed.
Seizure Control:
- Seizures may occur due to severe central nervous system involvement.
- Benzodiazepines like diazepam are the preferred treatment for cholinergic toxidrome seizures and also help reduce neuropathological damage.
Continuous Monitoring:
- Vital signs, heart rate, respiratory function, and neurological status should be closely observed.
- Monitoring neuromuscular function helps identify progression to respiratory failure requiring ventilatory support
These supportive therapies and monitoring protocols are described in established clinical toxicology guidelines and expert reviews.
Summary Table of Antidotes
| Antidote | Mechanism | Target Symptoms | Administration | Notes |
|---|---|---|---|---|
| Atropine | Muscarinic receptor antagonist | Muscarinic symptoms (secretions, bradycardia) | IV, titrated to effect | Does not affect nicotinic symptoms |
| Pralidoxime | Reactivates acetylcholinesterase | Nicotinic symptoms (muscle weakness) | IV, repeated boluses or infusion | Effective only before “aging” occurs |
cholinergic toxicity vs anticholinergic
Here is a comparison table summarizing the differences between cholinergic Toxicity vs anticholinergic:
| Feature | Cholinergic Toxicity | Anticholinergic |
|---|---|---|
| Cause | Excess acetylcholine stimulation | Blockade of acetylcholine receptors |
| Common Agents | Organophosphates, carbamates, nerve agents | Atropine, scopolamine, antihistamines, tricyclic antidepressants |
| Parasympathetic Effects | Overactivation (increased secretions, smooth muscle contraction) | Inhibition (decreased secretions, relaxation of smooth muscles) |
| Key Symptoms | Salivation, Lacrimation, Urination, Diaphoresis, GI cramps (SLUDGE) | Dry mouth, dry skin, urinary retention, constipation |
| Pupils | Constricted (miosis) | Dilated (mydriasis) |
| Heart Rate | Bradycardia | Tachycardia |
| Mental Status | Confusion, seizures in severe cases | Agitation, hallucinations, confusion, delirium |
| Treatment | Atropine (muscarinic antagonist), pralidoxime (enzyme reactivator) | Supportive care, physostigmine (antidote) |
This table helps clarify the opposing clinical features and treatments of the two toxidromes for effective diagnosis and management.
POV
The antidote for cholinergic toxicity involves a dual approach with atropine and pralidoxime. Atropine reverses the muscarinic effects by blocking muscarinic receptors, improving heart rate, reducing secretions, and alleviating bronchospasm. Pralidoxime addresses the biochemical cause by reactivating acetylcholinesterase, relieving nicotinic symptoms such as muscle weakness.
Early recognition of symptoms and prompt administration of these antidotes, combined with supportive care like oxygen and seizure management, are essential for successful treatment and reducing morbidity and mortality associated with this condition.
References
- Cholinergic Toxicity Overview. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK539783/
- Organophosphate Toxicity Management. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK470430/
- Cholinergic Toxicity Treatment Protocols – StatPearls. https://www.statpearls.com/point-of-care/305
- Organophosphate Toxicity Medication Guide. Medscape. https://emedicine.medscape.com/article/167726-medication